When you hear "generic medicine," you probably think of cheap pills that do the same thing as the brand-name version. But when it comes to biologics, that simple idea doesn’t hold up. Biologics aren’t made in a lab with chemicals-they’re grown in living cells. That makes them incredibly complex. And because of that, you can’t just copy them exactly. What you get instead is a biosimilar. And yes, it saves you money-but not in the way you might expect.
What’s the real cost difference?
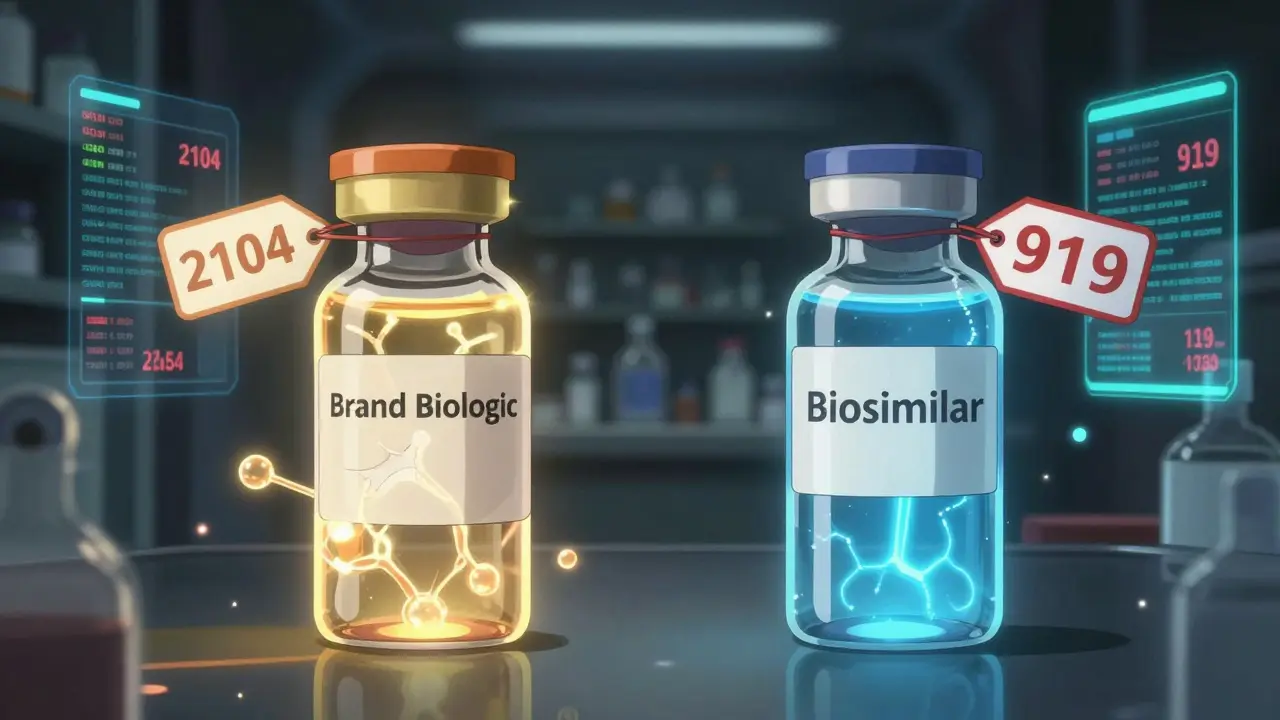
In 2025, a 30-day supply of a brand-name biologic in the U.S. costs around $2,104 on average. The biosimilar version? About $919. That’s more than half off. For patients paying out of pocket, that means savings of nearly 23% just by switching. And it gets better: once biosimilars enter the market, the original brand often drops its price too. In fact, after biosimilars hit the market, brand biologics have lowered their prices by an average of 25-33%. Take Humira, for example. Before biosimilars arrived, it cost about $80,000 per patient per year in the U.S. After patents expired in 2023, biosimilars like Hyrimoz came in at 80% less. Today, they hold 65% of the market. That’s not just a discount-it’s a market reset.Why aren’t biosimilars cheaper like regular generics?
Regular generics? They’re chemically identical to their brand-name counterparts. That’s why you can buy a $4 version of a common blood pressure pill. But biologics? They’re made from living organisms-cells, proteins, antibodies. No two batches are exactly alike. So regulators don’t call them "generics." They’re called biosimilars, meaning they’re highly similar, but not identical. And because they’re so complex to make, they cost way more to develop. Developing one biosimilar can cost between $100 million and $250 million. Compare that to a regular generic, which might cost under $5 million. That’s why biosimilars don’t drop to 90% off like traditional generics. They launch at 40-50% off, and even that’s a huge win.How much have biosimilars saved the system?
Since 2015, biosimilars have saved the U.S. healthcare system between $36 billion and $56 billion, depending on who’s counting. In 2024 alone, savings hit $12-20 billion. And here’s the kicker: generic and biosimilar drugs together saved $445 billion in 2023. That’s because generics cover 90% of all prescriptions-but only 13% of total drug spending. The math is simple: if 90% of your pills are cheap, but they’re only 13% of the bill, that means the other 10% of drugs (mostly biologics) are eating up 87% of the money. That’s why biosimilars are so critical. They’re the only tool we have to bring down the cost of the most expensive medicines.
Why aren’t more people using them?
Here’s the problem: even though biosimilars are approved, safe, and cheaper, they’re still underused. Only about 15-20% of eligible patients are on them. Why? First, big drug companies use patent thickets-layers of patents that delay biosimilar entry. They file dozens of minor patents to stretch protection beyond the original 12 years. It’s legal, but it’s designed to block competition. Second, Pharmacy Benefit Managers (PBMs) get paid through rebates. If a brand biologic gives them a 30% rebate, they have no incentive to push the cheaper biosimilar, even if it’s better for the patient. The system rewards high prices, not savings. Third, doctors and patients are still unsure. Many think biosimilars are "second-rate." But the FDA says they’re just as safe and effective. In fact, over 76 biosimilars are approved in the U.S. as of October 2025, and none have shown lower effectiveness in real-world use.What’s changing in 2026?
The FDA just released new draft guidance to make biosimilar development faster and cheaper. They’re cutting unnecessary clinical trials and letting companies use real-world data instead. That could cut development time by years and costs by millions. The Biden administration’s Biosimilars Action Plan is pushing for better reimbursement rules. Medicare and Medicaid are starting to prioritize biosimilars in formularies. Some health plans now require patients to try a biosimilar before covering the brand. And the market is responding. By 2030, analysts expect biosimilar use to jump from 20% to 35-40%. That could mean $125 billion in annual savings-just from these drugs.
What does this mean for you?
If you’re on a biologic-like Humira, Enbrel, or Rituxan-ask your doctor if a biosimilar is an option. It’s not a gamble. It’s a proven, FDA-approved alternative. You could save thousands a year. Even if your insurance doesn’t push it, you can still request it. Many pharmacies stock biosimilars and will fill them if you ask. And if you’re paying cash? The savings are even more dramatic. Some biosimilars cost less than $300 a month. That’s a quarter of the brand price. For chronic conditions like rheumatoid arthritis or Crohn’s disease, that’s life-changing.Don’t let myths stop you
Some people think biosimilars are "inferior" because they’re not exact copies. But that’s like saying a hand-made leather shoe isn’t as good as the original because it’s stitched differently. It’s still a shoe. And if it lasts just as long, costs half as much, and doesn’t hurt your feet-why wouldn’t you choose it? The science is clear: biosimilars work. The data is clear: they save money. The question isn’t whether they’re safe. It’s why we’re not using them more.What’s next?
More biologics are coming off patent in the next five years. Drugs for cancer, diabetes, and multiple sclerosis will soon have biosimilar options. If we fix the broken incentives-stop the patent games, cut PBM rebates, educate prescribers-we could save hundreds of billions over the next decade. Right now, biosimilars are the best tool we have to fix the broken cost of modern medicine. They’re not perfect. But they’re better than what we have.Are biosimilars the same as generics?
No. Generics are exact chemical copies of small-molecule drugs, like aspirin or metformin. Biosimilars are highly similar but not identical versions of complex biologic drugs made from living cells. They’re not exact copies because biologics are too complex to replicate perfectly-but they work the same way and are just as safe.
Are biosimilars safe?
Yes. The FDA requires biosimilars to show no clinically meaningful differences in safety, purity, or potency compared to the brand-name biologic. Over 76 are approved in the U.S., and real-world data from millions of patients shows no increased risk. Many patients switch without any issues.
Why are biosimilars still expensive?
Because they’re hard to make. Developing a biosimilar costs $100-250 million-far more than a regular generic. Plus, brand companies use patent tactics and rebate deals to slow adoption. That keeps prices higher than they could be. But even with those barriers, biosimilars still cost 40-80% less than the original.
Can I ask my doctor for a biosimilar?
Absolutely. You have the right to ask if a biosimilar is available and appropriate for your condition. Many doctors aren’t familiar with them, so you might need to bring it up. If your insurance covers the brand but not the biosimilar, ask them to change their policy-patient demand drives change.
Will switching to a biosimilar affect my treatment?
For most patients, no. Clinical trials and real-world use show that switching from a brand biologic to a biosimilar doesn’t reduce effectiveness or increase side effects. Some patients even report better tolerance because they’re on a lower-cost version that’s covered better by insurance.
How do I know if my drug has a biosimilar?
Check the FDA’s biosimilar product list online or ask your pharmacist. Common biologics with biosimilars include Humira (adalimumab), Enbrel (etanercept), Rituxan (rituximab), Herceptin (trastuzumab), and Avastin (bevacizumab). If your drug is one of these, ask if a biosimilar version is available.

10 Comments
Write a comment